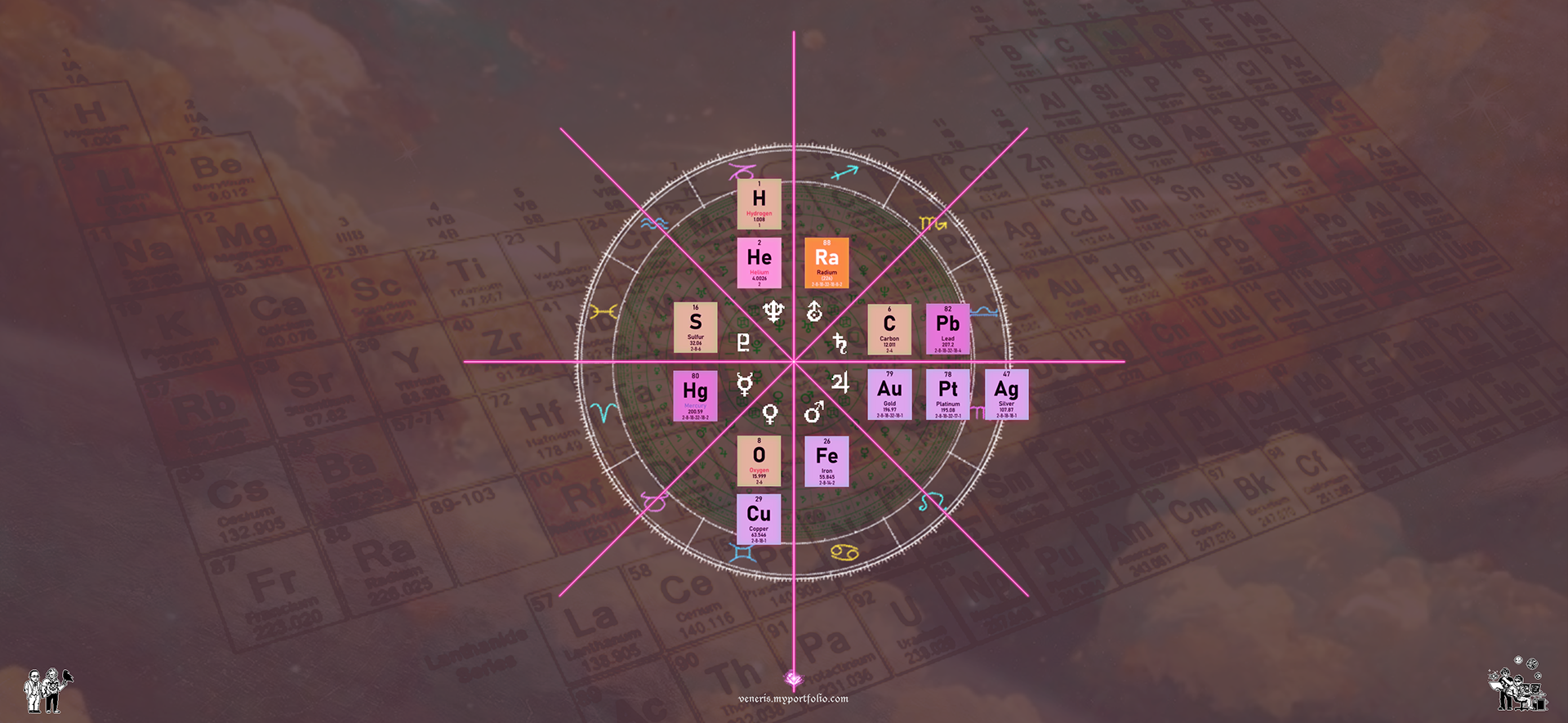
Elements arranged based on Johndro's Planetary Associations
The History of the Periodic Table
Antoine Lavoisier
tried grouping the elements as metals and nonmetals.
Johann Wolfang Döbereiner
arranged physical & chemical properties in groups of three in increasing order of atomic weight and called them triads.
John Newlands
the first to arrange the elements into a periodic table with increasing order of atomic masses. He found that every eight elements had similar properties and called this the law of octaves. He arranged the elements in eight groups but left no gaps for undiscovered elements.
Dmitri Mendeleev
created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight. Mendeleev predicted the properties of some undiscovered elements and gave them names such as "eka-aluminium" (now called galium)
Lothar Meyer
produced a version of the periodic table similar to Mendeleev’s in 1870. He left gaps for undiscovered elements but never predicted their properties.
Henry Moseley
used X-rays to measure the wavelengths of elements and correlated these measurements to their atomic numbers. He then rearranged the elements in the periodic table on the basis of atomic numbers.
tried grouping the elements as metals and nonmetals.
Johann Wolfang Döbereiner
arranged physical & chemical properties in groups of three in increasing order of atomic weight and called them triads.
John Newlands
the first to arrange the elements into a periodic table with increasing order of atomic masses. He found that every eight elements had similar properties and called this the law of octaves. He arranged the elements in eight groups but left no gaps for undiscovered elements.
Dmitri Mendeleev
created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight. Mendeleev predicted the properties of some undiscovered elements and gave them names such as "eka-aluminium" (now called galium)
Lothar Meyer
produced a version of the periodic table similar to Mendeleev’s in 1870. He left gaps for undiscovered elements but never predicted their properties.
Henry Moseley
used X-rays to measure the wavelengths of elements and correlated these measurements to their atomic numbers. He then rearranged the elements in the periodic table on the basis of atomic numbers.



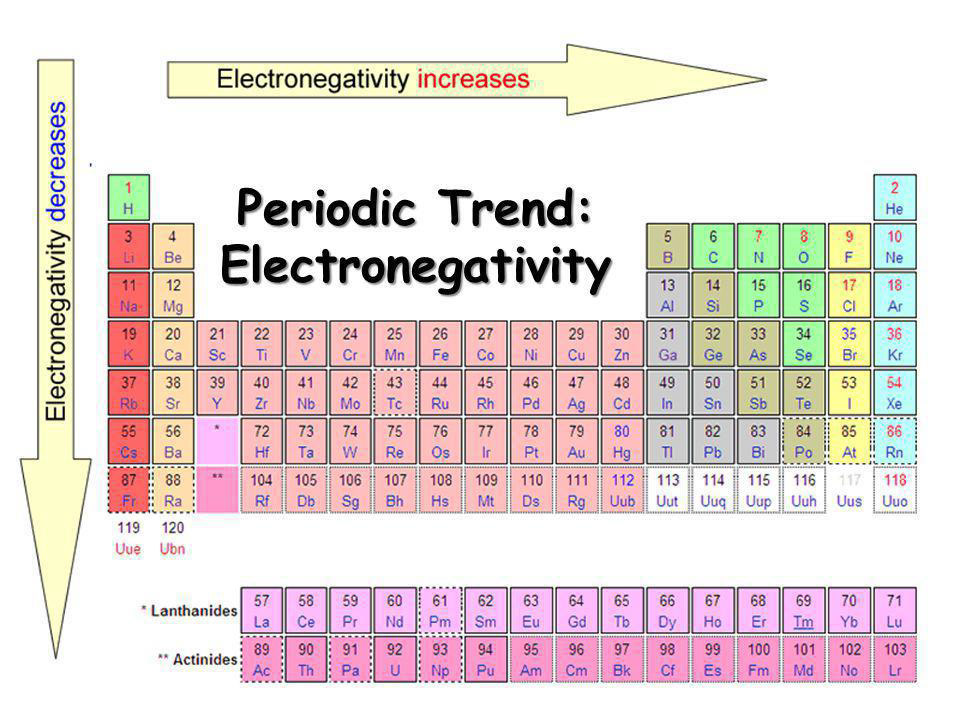
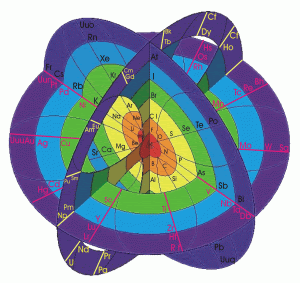
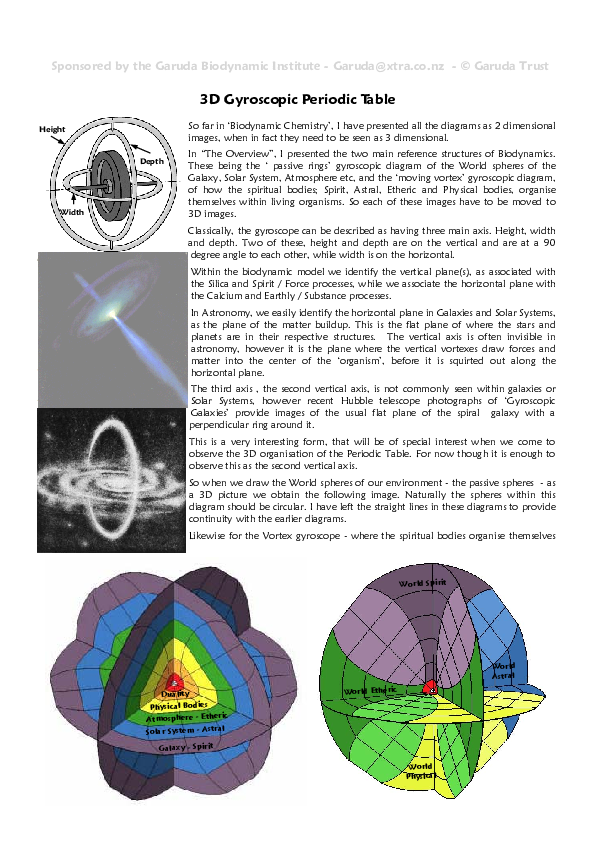






Feel free to check out these diagrams
Copyright © 2024 by Ian Murphy
All rights reserved. This work, or parts thereof, may not be reproduced in any form without permission in writing from the author.
All rights reserved. This work, or parts thereof, may not be reproduced in any form without permission in writing from the author.